In a Combustion Reaction One of the Reactants Is Always
Which of the following statements is true about the decomposition of a simple binary compound. The reactant must always be a molecular substance.

Stoiciometry Practice Quiz 11 3
O2 will always be one of the reactants and the products will always be H2O and CO2.

. The reactant is a single substance. A the reactants are generally two ionic compounds in aqueous solution. 2 pts Question 2 Combustion reactions are characterized by water always being one of the products oxygen always being one of the reactants carbon dioxide always being one of the products carbon always being one of the reactants.
The products cannot be predicted. The reactant must always be a molecular substance. Fri Sep 28 2018 723 am.
Post by Amy Dinh 1A Wed Oct 03 2018 922 pm. Exothermic reactions are reactions that absorb heat from the surroundings. For a specific hydrocarbon say hexanes.
Oxygen alw View the full answer Transcribed image text. C In a decomposition reaction. True or False 3.
The final products of combustion always include an oxide and the release of heat energy. The other reactant usually contains carbon and hydrogen and sometimes oxygen too but only if the non-oxygen reactant is a hydrocarbon. B energy in the form of heat or light is often produced.
Combustion reactions are always endthermic 2. The products cannot be predicted. Oxygen gas must be one of the reactants carbon monoxide will be produced the reaction absorbs energy one reactant must contain chlorine.
D both reactants always being elements. Alkane oxygen rarr Carbon dioxide and water. Common examples of combustion that we see every day are.
How do you know. C Cd NO32 Na2S CdS 2 NaNO3. From a hydrocarbon the products are ALWAYS carbon dioxide and water under conditions of complete combustion.
Every combustion reaction we deal with produces gas with oxygen in the product so O2 oxygen gas must be a reactant. The reactant is a single substance. Yes Oxygen is always needed for a combustion reaction to occur and the products will always have CO2 and H2O.
B water always being one of the products. Combustion reactions always produce visible light. CH4 l O2 g - C02.
The reactants in a combustion reaction are always hydrocarbons and oxygen. And similar reactions can occur in different chemicals that in one case resemble combustion and in another case do not but they are chemically similar. In a combustion reaction one of the reactants is always ____.
D 2 Mg O2 2 MgO. C the reactants are usually a metal and a nonmetal. It is known that combustion is defined as the reaction of any substance in the presence of oxygen which is accompanied by generation of heat and light energy.
Chemistry questions and answers. True or False 2. C_6H_14l 192O_2g rarr 6CO_2 7H_2Ol Is the equation above balanced.
Combustion reactions are characterized by. In a combustion reaction one of the reactants is always. As the name suggests a complete combustion reaction fully utilises.
D one of the reactants is often water. Complete combustion of propane gas. Combustion reactions consist of fuel and oxygen.
Combustion reactions are characterized by A oxygen always being one of the reactants. If heptane C_7H_16 were combusted what. In any combustion reaction there will always be formation of water vapor.
Does a combustion reaction always need to have an organic compound and oxygen gas as the reactants and water and Carbon dioxide as the product. Thus combustion always include oxygen as one of the reactant. C carbon dioxide always being one of the products.
Amy Dinh 1A Posts. Which of the following statements is true about the decomposition of a simple binary compound. In a combustion reaction one of the reactants is always ____.
True or False 4. Hydrocarbons are the fuel and oxygen is necessary for combustion. What is always true of a combustion reaction.
The products of combustion reactions are water and carbon dioxide. All combustion reactions produce the same amount of energy. Reaction of combustion of methane will give off lot of energy.
For example methane reacts with Oxygen in this way. If the other reactant aside from oxygen is an organic compound the products of a complete combustion reaction are water and carbon dioxide.
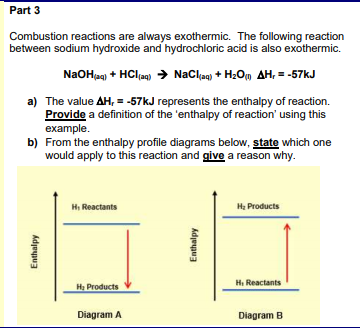
Solved Part 3 Combustion Reactions Are Always Exothermic Chegg Com

1 Give The Formulas To Show The Reactants And The Products
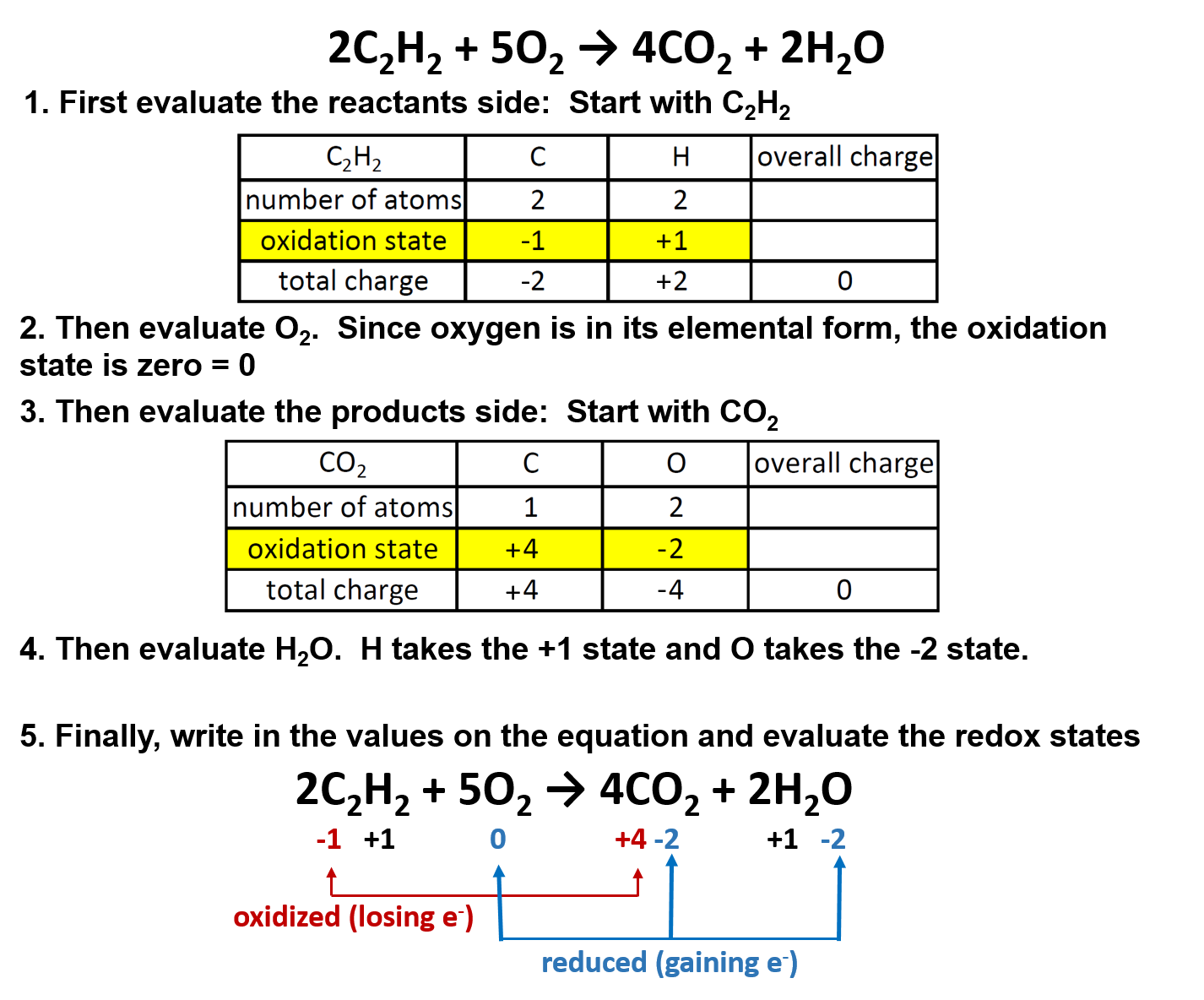
Ch104 Chapter 5 Chemical Reactions Chemistry

Answers 2 C 13 C 3 B 14 D 4 C 15 D 5 B 16 D 6 A 17 A 7 C 18 D 8 A 19 B 9 D 20 D 10 A 21 A 11 B 22 B 12 C Ppt Download

Chemical Reactions Tutorial Ppt Download

In A Combustion Reaction One Of The Products Of A Chegg Com

Chapter 11 Review Chemical Reactions Balance Fe Cl 2 Fecl 3 In A Combustion Reaction One Of The Reactants Is Ppt Download

Solved 2 Pts Question 2 Combustion Reactions Are Chegg Com
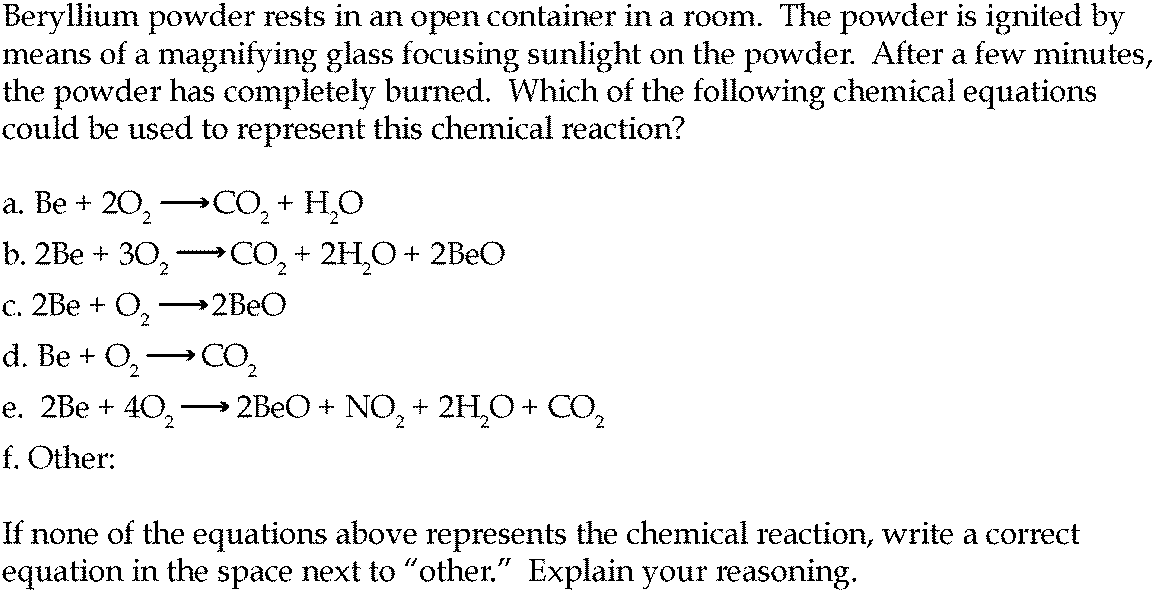
Combustion Always Produces Carbon Dioxide And Water A Discussion Of University Chemistry Students Use Of Rules In Place Of Principles Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C4rp00089g

Balancing Another Combustion Reaction Video Khan Academy

Solved Combination Decomposition And Combustion Reactions Chegg Com

Test Review Rancho High School

How To Predict Products For Combustion Reactions Including Hydrocarbon Combustion Youtube

Chapter 11 Review Chemical Reactions Ppt Download





Comments
Post a Comment